How I work
A rigorous, transparent and collaborative approach to scientific consulting
For more than 30 years, I have worked at the intersection of science, regulation and strategic decision-making. My approach is built on three principles:
- Scientific integrity – Evidence-based, methodologically sound, aligned with the latest research.
- Clarity – Complex concepts translated into clear, actionable guidance for decision-makers.
- Partnership – Working closely with your team to support informed, confident choices.
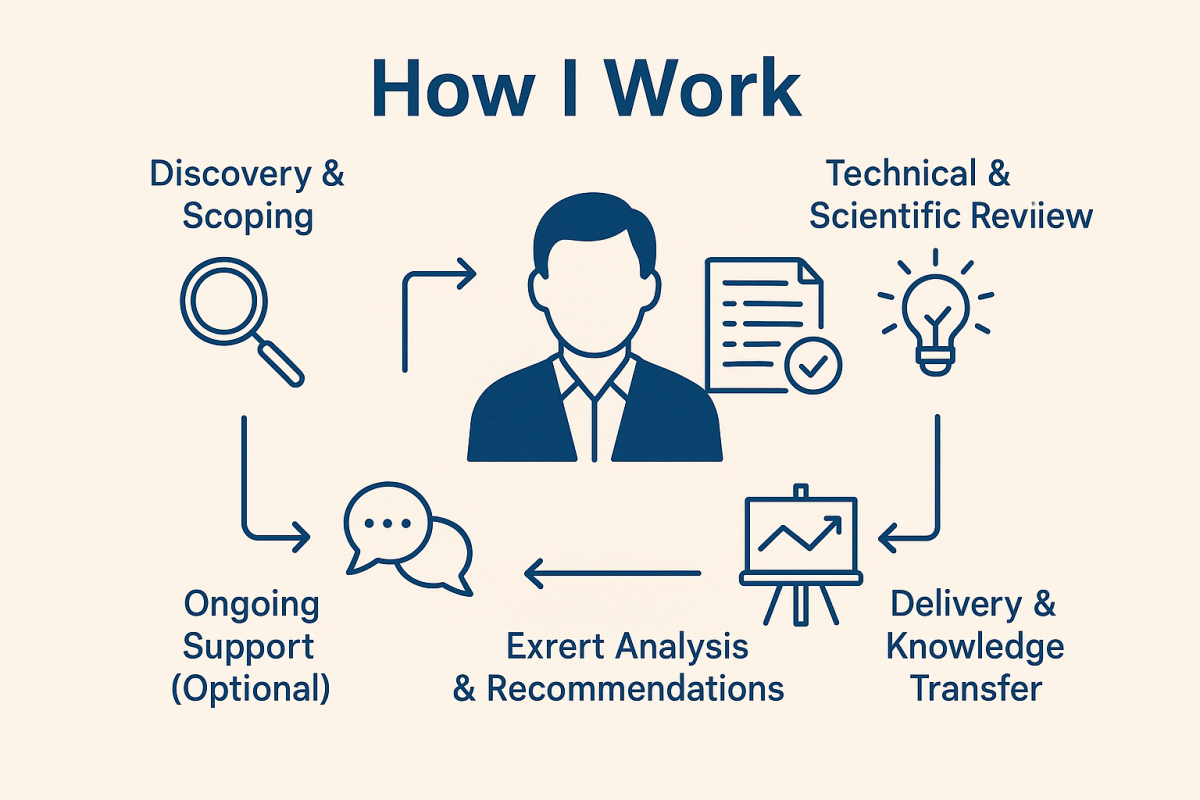
My 5-step process
1. Discovery & Scoping
We begin with a focused conversation to understand your goals, context and constraints.Together we clarify:
- Your scientific and strategic priorities
- The questions you need to answer
- The scope, timelines and key stakeholders
- The scientific inputs and data available
2. Technical & Scientific Review
I conduct a rigorous analysis of the materials you provide:
- Available data and studies (toxicology, LCA, exposure, safety)
- Existing assessments, models or reports
- Methodological choices and assumptions
- Compliance with SSbD, LCA/RA standards or EU expectations
3. Expert Analysis & Recommendations
Based on the review, I develop a clear, concise set of conclusions and next steps:
- What the science supports
- Where uncertainties lie
- What needs refinement or further evidence
- How to align with best practices and EU requirements (SSbD, REACH, PEF, EFSA, ECHA, Horizon Europe)
4. Delivery & Knowledge Transfer
You receive a high-quality deliverable, adapted to your needs:
- Expert review note
- Technical memo
- Slide deck for internal decision-making
- Risk or LCA/RA guidance
- Text for proposal sections
- Recommendations for next steps or further analysis
5. Ongoing Support (Optional)
For longer engagements, I can continue supporting your project through:
- Iterative reviews
- Participation in technical meetings
- Advisory support during Horizon Europe implementation
- Coaching for R&D or proposal teams
- Scientific QA for deliverables and evidence packages
A partnership built on trust and rigour
I work with a limited number of clients to provide the depth, attention and precision that high-stakes scientific work requires.
Direct access to an expert
No junior staff, no hand-offs — you work directly with me from start to finish.
Clear, actionable advice
Every recommendation is grounded in science and tailored to real-world constraints.
European-level insight
My experience at the European Commission’s Joint Research Centre, Horizon Europe evaluation, and industry projects provides a perspective that bridges science, policy and implementation.
Let’s Work Together
If you need structured guidance, a high-quality scientific review, or a partner to support your SSbD, LCA/RA or Horizon Europe activities: